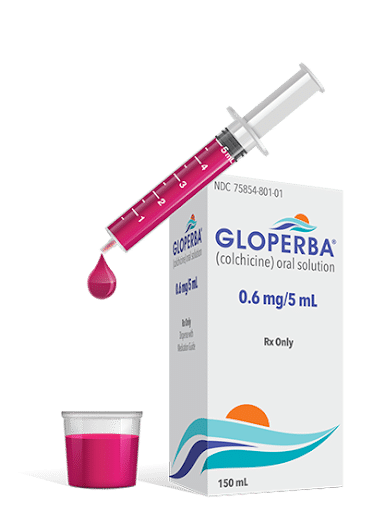
- Lanched Q2:24 by Romeg commercial partner Scilex Pharmaceuticals
- For more details, visit www.gloperba.com
- Only FDA approved oral solution for the prophylactic treatment of gout flares in adults
- 0.6mg/5mL Colchicine
- Liquid dosage form provides dosing flexibility